Single-molecule FRET uncovers hidden conformations and dynamics of human Argonaute2
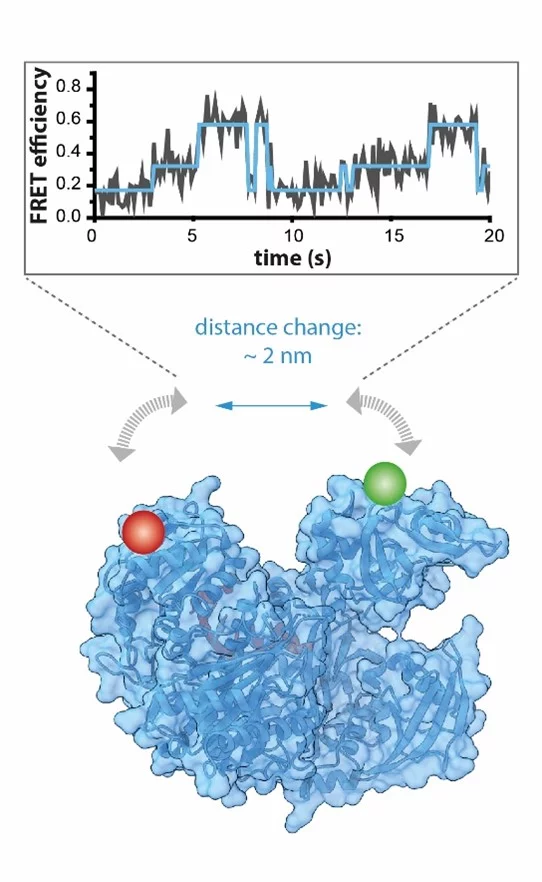
Human Argonaute2 (hAgo2) is a key player of posttranscriptional regulation of gene expression and raised remarkable scientific and biotechnological interest with far-reached implications for RNA-based therapy. It binds short guide nucleic acids to down-regulate complementary target messenger RNAs. To proceed within its catalytic cycle and fine-tune regulation, hAgo2 undergoes a variety of conformational changes. To gain an in-depth understanding of the mechanistic complexity and dynamics of hAgo2 researchers at the RUN performed time-resolved single-molecule FRET measurements – a method that allows distance measurements in the subnanometer range. To this end, native hAgo2 was site-specifically fluorescently labelled using cutting-edge biochemical technologies based on the incorporation of unnatural amino acids with unique reactive sidechains suitable for site-specific reactions into human proteins. Combining biochemical and biophysical expertise in one lab ultimately allowed the team led by Prof. Dina Grohmann and Dr. Sarah Willkomm to monitor conformational changes of hAgo2 in real-time with millisecond time resolution. This study significantly expanded the knowledge about the structural and mechanistic framework of hAgo2 throughout its catalytic cycle showing how conformational heterogeneity contributes to the functionality and regulation of hAgo2 and hAgo2/RNA complexes.
Press release (idw) in German.